Gavi and UNICEF Strike Major Deal to Expand Access to R21 Malaria Vaccines Globally
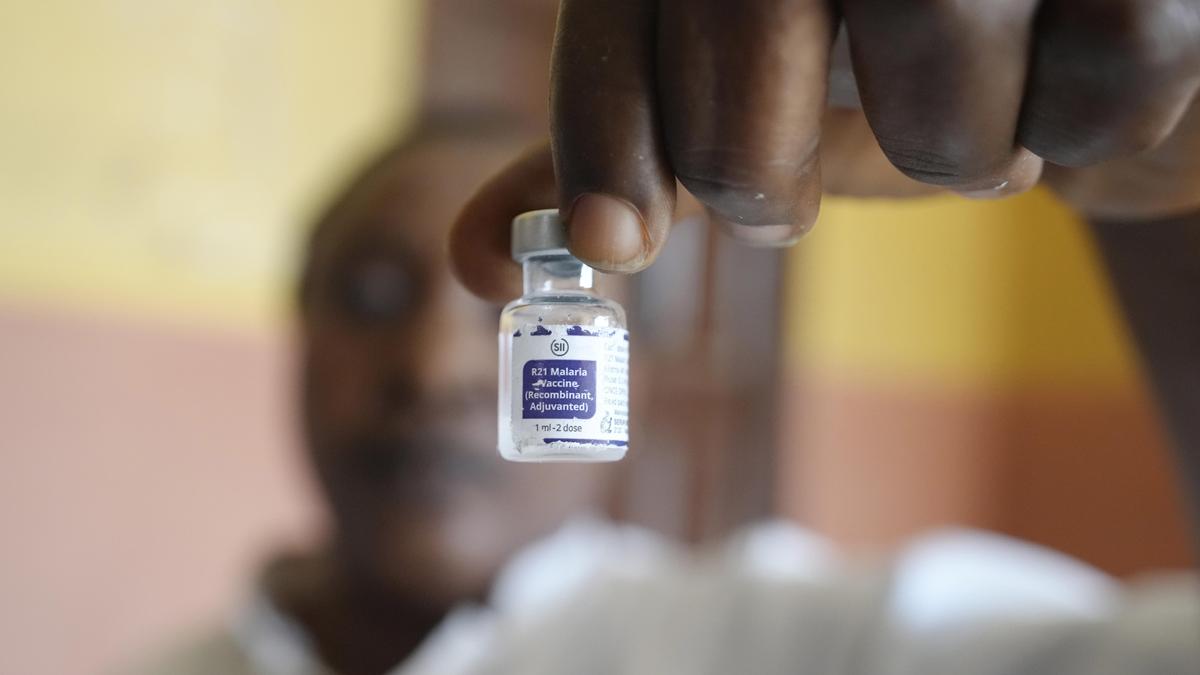
GENEVA — Gavi, the Vaccine Alliance, and UNICEF announced Tuesday a new agreement to expand access to the R21/Matrix-M malaria vaccine, marking a significant step in global efforts to curb a disease that kills hundreds of thousands of people each year, mostly young children in Africa. The deal, finalized this week, is intended to increase supply, reduce costs and speed delivery of the vaccine to countries with the highest burden of malaria.
A Milestone for Malaria Prevention
The agreement enables UNICEF, the world’s largest vaccine buyer, to procure doses of the R21/Matrix-M vaccine on behalf of participating Gavi countries. Developed by Oxford University and manufactured by the Serum Institute of India, the R21 vaccine received World Health Organization recommendation in late 2023 after clinical trials showed promising efficacy.
“This is a major turning point in the fight against malaria,” said Aurélia Nguyen, Gavi’s chief program officer. “Expanding access to a safe, effective, affordable vaccine gives countries a powerful new tool to protect millions of children.”
UNICEF said the new procurement mechanism will allow countries to place orders immediately as supply ramps up. The first shipments are expected to begin in 2025, with several African nations already signaling interest in early rollout.
Why the Agreement Matters Now
Malaria remains one of the world’s most persistent infectious diseases, causing an estimated 249 million cases and 608,000 deaths in 2022, according to the WHO. Children under age 5 account for roughly 80 percent of malaria deaths in Africa, where the disease is endemic.
Global progress against malaria has stalled in recent years due to climate-driven changes in mosquito patterns, increasing resistance to insecticides and reduced funding for prevention efforts. The introduction of the R21 vaccine, alongside the RTS,S/AS01 malaria vaccine already in use, is expected to help close the immunity gap.
UNICEF Executive Director Catherine Russell said the agreement reflects “a shared commitment to ensure no child dies from a preventable disease simply because of where they are born.”
How the R21 Vaccine Works
The R21/Matrix-M vaccine is considered a next-generation version of earlier malaria vaccines. It uses a fragment of the malaria parasite combined with Novavax’s Matrix-M adjuvant to stimulate a stronger immune response.
Phase III trial data published last year found the vaccine to be between 66 percent and 80 percent effective in preventing clinical malaria when multiple doses were administered before peak transmission season. Its lower cost — estimated at around $2 to $4 per dose — is expected to make it more accessible to low-income countries.
The Serum Institute of India, the world’s largest manufacturer of vaccines by volume, has said it can produce more than 100 million doses per year initially, with the possibility of scaling up to 200 million doses. Officials say supply capacity was a key factor in securing Tuesday’s agreement.
Complementing Existing Malaria Tools
Health experts caution that vaccines alone will not eliminate malaria but say the new agreement strengthens an integrated strategy that includes bed nets, seasonal chemoprevention and rapid diagnostic testing.
“For the first time, we have two malaria vaccines available at global scale,” said Dr. Mary Hamel, head of the WHO’s malaria vaccine implementation program. “This gives countries flexibility and reliability in planning immunization schedules.”
Some countries anticipate using both RTS,S and R21 vaccines in different regions or seasons depending on supply, transmission patterns and local health systems.
Country Perspectives and Early Interest
Several African governments have welcomed the announcement, noting that the new agreement helps address chronic challenges in obtaining consistent vaccine supply.
Ghana, Nigeria and Burkina Faso — the first countries to approve the R21 vaccine domestically — are expected to be among the early recipients once UNICEF begins distribution. Health officials in these countries said the agreement offers hope of achieving broader coverage than previously possible with only one WHO-recommended malaria vaccine available.
“Reliable access to R21 will be critical as we expand malaria vaccination into rural communities,” said Dr. Emmanuel Toure, a public health adviser with Burkina Faso’s immunization program. “This is the most important development in malaria prevention in decades.”
Financial Considerations and Affordability
Gavi confirmed that the alliance will provide co-financing support for eligible low-income countries, continuing its standard tiered assistance model. The organization has allocated hundreds of millions of dollars to malaria vaccine rollout through 2025, though final distribution costs depend on country uptake and supply availability.
The lower production cost of R21 compared with RTS,S could reduce long-term procurement expenses for global partners. UNICEF officials said affordability and stable manufacturing capability were considered essential in negotiating Tuesday’s agreement.
“Predictable pricing helps countries plan their immunization budgets more effectively,” said Jean-Marie Vianney, UNICEF’s chief of global supply operations. “This agreement reflects a commitment to financial sustainability as well as public health impact.”
Challenges Ahead
Despite optimism, officials caution that significant challenges remain. Many high-burden regions lack infrastructure for cold-chain storage, community outreach and health worker training, all of which are necessary for a successful vaccine rollout. Funding shortfalls in global malaria programs also persist.
Health experts say climate change may further complicate efforts by expanding mosquito habitats into new regions and lengthening transmission seasons.
To address these issues, Gavi and UNICEF plan to collaborate closely with national health ministries to strengthen delivery systems and community engagement campaigns. They also aim to coordinate with the WHO, the Global Fund and regional malaria programs to prevent duplication of resources.
A Step Toward Long-Term Malaria Control
International health organizations say the agreement represents a strategic step toward reducing global malaria deaths by at least 90 percent by 2030 — an ambitious target set by the WHO’s Global Technical Strategy.
Early modeling studies suggest that widespread use of malaria vaccines could prevent tens of millions of cases per year if implemented at scale.
“This agreement moves us closer to a world where no child dies from malaria,” Nguyen said. “But vaccines must be part of a sustained, long-term commitment by all global partners.”
As global demand grows, Gavi and UNICEF say they will continue exploring partnerships with manufacturers and donors to maintain supply and support further innovation in malaria prevention.
